Abstract
Introduction: Mantle cell lymphoma (MCL) is an aggressive form of non-Hodgkin lymphoma and is typically managed using chemoimmunotherapy and oral targeted therapies. However, there is a paucity of research examining the real-world treatment patterns and outcomes in the MCL population in community setting.
Methods: Adults with MCL who initiated treatment between 1 January 2013 and 31 October 2016 were identified retrospectively from electronic health records (EHR) of McKesson Specialty Health and followed up until 30 April 2017. Patients in clinical trials were excluded. Demographic, disease and treatment characteristics were abstracted from structured data elements of the EHR. Overall survival (OS) was analyzed using Kaplan-Meier analysis and time to next treatment (TTNT: time from 1L treatment completion to 2L treatment initiation) was analyzed using descriptive statistics.
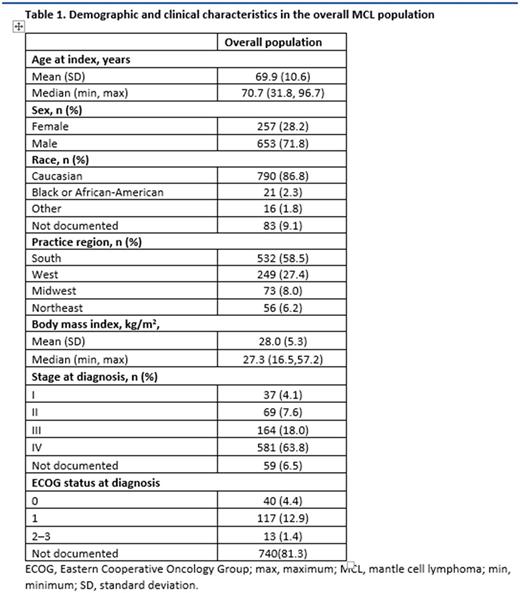
Results: In total, 910 patients, with a median follow-up of 17.6 mos (range, 0.03-51.8), were included. Median age at treatment initiation was 70.7 years; 71.8% were male and 68.3% were initially diagnosed with stage IV MCL (Table 1). Of patients (n=170) with a known Eastern Cooperative Oncology Group (ECOG) score at diagnosis, 23.5% and 68.8% had an ECOG performance status of 0 and 1, respectively. Frequent 1L treatments included bendamustine + rituximab (41.0%) with a median treatment duration of 20.2 weeks (range, 0.1-220.1 weeks), rituximab monotherapy (17.6%) with a median treatment duration of 29.6 weeks (range, 0.1-216.2 weeks) and rituximab + cyclophosphamide + doxorubicin + vincristine +/- prednisone (R-CHO+/-P, 13.4%) with a median treatment duration of 16.7 weeks (range, 0.1-144.4 weeks). Of the 796 patients treated with a rituximab-containing regimen in the 1L, 168 patients (21.1%) were identified as having rituximab maintenance following 1L treatment.
A total of 208 patients (22.9%) were treated further in the 2L setting with the most common regimens: ibrutinib monotherapy (18.3%), bendamustine + rituximab (14.4%), and rituximab monotherapy (11.1%). Median TTNT was 57.1 weeks (range 0.1-224.5 weeks) in the overall population who received 2L treatment.
Median OS from 1L treatment initiation was not reached in the overall 1L treated population. OS did not differ by regimens utilized in the 1L setting (log-rank P=.2503). Survival probabilities at 50 months were 76.8% for patients initiating bendamustine + rituximab, 79.7% for rituximab monotherapy, 72.6% for R-CHO+/- P therapy.
Conclusions: This is the first real-world study evaluating treatment patterns and outcomes in MCL in a large community oncology network. The most common frontline regimens utilized were bendamustine + rituximab and rituximab monotherapy with survival not differing by 1L regimen. Additional analyses evaluating treatment-related adverse events and associated outcomes for infused and oral treatments by line of therapy are ongoing.
Sharman: Novartis: Research Funding; Genentech: Research Funding; Gilead Sciences, Inc.: Consultancy, Honoraria, Research Funding, Speakers Bureau. Black-Shinn: McKesson Specialty Health: Employment. Clark: McKesson Specialty Health: Employment, Equity Ownership. Karve: AstraZeneca: Employment, Other: Stocks.
Author notes
Asterisk with author names denotes non-ASH members.